Introducing CITIDARON®
How CITIDARON® works
When to use CITIDARON®?
Why use CITIDARON®?
How to use CITIDARON®?
CITIDARON® prescribing considerations
WHY USE CITIDARON®?
- The active ingredient of CITIDARON® (cytisine) has been used as a cessation treatment in Eastern Europe for decades1
- A meta-analysis that included 7 studies of cytisine published between 1968 and 2011 showed that cytisine was an effective treatment for smoking cessation (risk ratio = 1.59, confidence interval 1.43 to 1.75)2
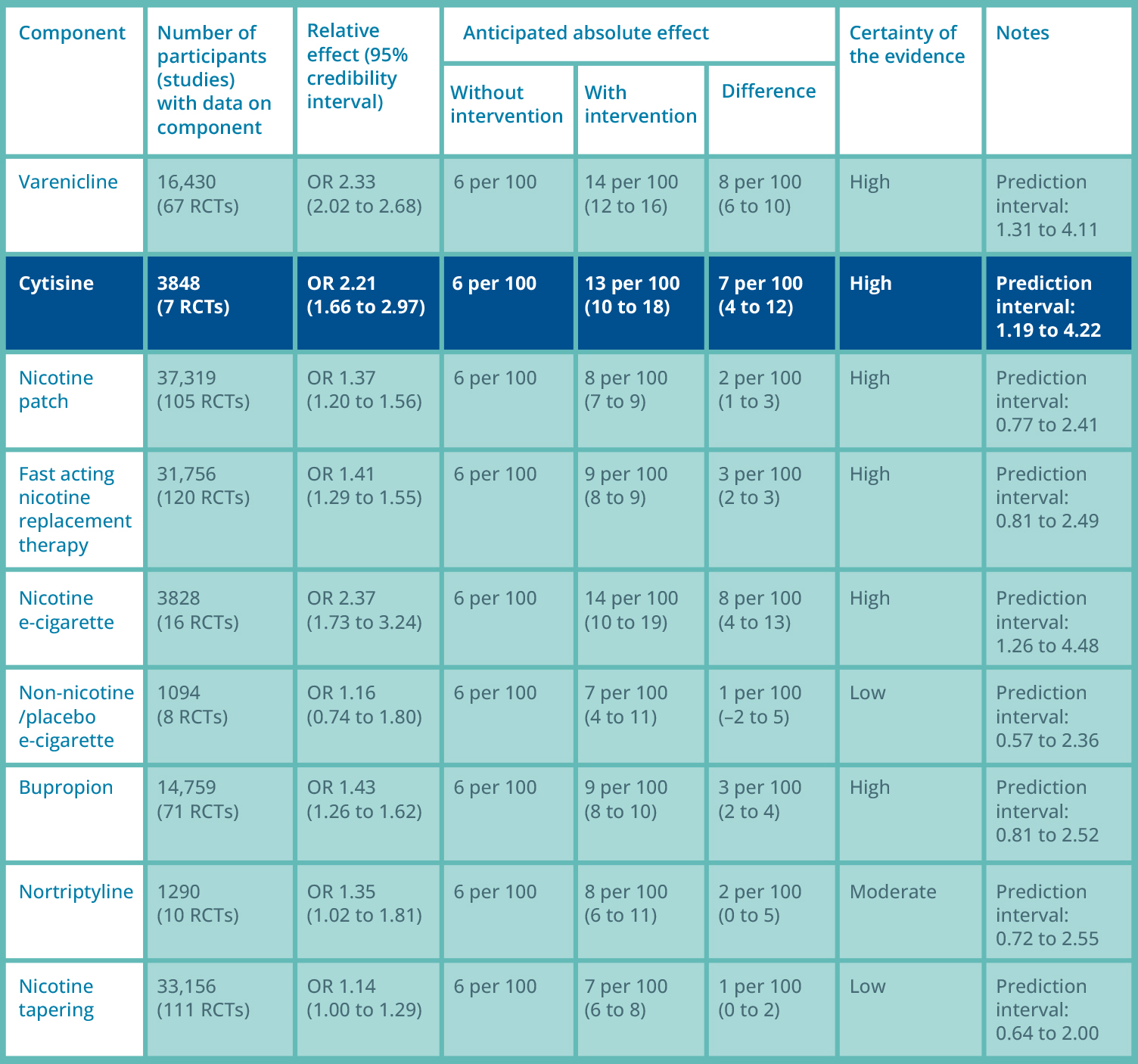
In a recent Cochrane review, the chances of quitting smoking with cytisine are over twice as high, with a success rate of 13%, compared to a 6% chance with control predominantly at 6 months to 12 months.3
- Cytisine exhibited a low frequency of serious adverse events during clinical trials, suggesting it is generally well-tolerated by patients.
- The high-certainty evidence from the study underlines a well-defined safety profile for Cytisine,making it a predictable option for smoking cessation.
Please note that some of the studies referenced in the Cochrane report used dosage regimes outside the licensed dosage regime.
Summary of findings: Lindson meta-analysis, smoking cessation at 6 months 3
RCT: randomised controlled trial; OR; odds ratio.
Please note some of the studies referenced in the Cochrane report used dosage regimes outside the licensed dosage regime
References
- Karnieg T, Wang X. CMAJ 2018;190(19):E596.
- Hajek P, McRobbie H, Myers K. Thorax 2013;68:1037-1042.
- Lindson N, Theodoulou A, Ordóñez-Mena JM et al. Cochr Data Syst Rev 2023, Issue 9. Art. No.: CD015226.

Pharmacovigilance or Medical enquiries please contact:
E: drugsafety@consilienthealth.com
T: (01) 205 7766
All other queries: (01) 205 7760
Healthcare professionals are asked to report any suspected adverse reactions. To report an adverse event or a product complaint about a Consilient Health medicine, please contact Consilient Health at drugsafety@consilienthealth.com or 012057766. Adverse events and product complaints may also be reported to the Health Products Regulatory Authority. Reporting form and information can be found at http://www.hpra.ie then click on “report an issue”.
IE-CH-1562 Date of Preparation April 2024