Introducing CITIDARON®
How CITIDARON® works
When to use CITIDARON®?
Why use CITIDARON®?
How to use CITIDARON®?
CITIDARON® prescribing considerations
CITIDARON® PRESCRIBING CONSIDERATIONS
CITIDARON® INDICATION1
- CITIDARON® is indicated for smoking cessation and reduction of nicotine cravings in smokers who are willing to stop smoking
- The treatment goal of CITIDARON® is the permanent cessation of the nicotine-containing products use
- Please refer to the full Summary of Product Characteristics before prescribing CITIDARON®
CONTRAINDICATIONS 1
CITIDARON® is contraindicated in patients with:
- Hypersensitivity to the active substance of any of excipients
- Unstable angina
- A history of recent myocardial infarction
- Clinically significant arrhythmia
- A history of recent stroke
- Pregnancy or breastfeeding
CITIDARON® should be used with caution in patients with:
- Ischaemic heart disease
- Heart failure
- Hypertension
- Pheochromocytoma
- Atherosclerosis and other peripheral vascular disease
- Gastric and duodenal ulcer
- Gastroesophageal reflux disease
- Hyperthyroidism
- Diabetes
- Schizophrenia
- Depressed mood may be a symptom of nicotine withdrawal. Clinicians should be aware of the possible emergence of serious neuropsychiatric symptoms in patients attempting to quit smoking with and without treatment
- Patients should be aware that the simultaneous administration of CITIDARON® and smoking or use of products containing nicotine could lead to aggravated adverse reactions of nicotine
- Women of childbearing potential must use highly effective contraception while taking CITIDARON®. It is currently unknown whether CITIDARON® may reduce the effectiveness of systemically acting hormonal contraceptives, and therefore women using systemically acting hormonal contraceptives should add a second barrier method
- There is no clinical experience of CITIDARON® in patients with renal or hepatic impairment, therefore CITIDARON® is not recommended for use in this patient population
- CITIDARON® is not recommended for use in patients <18 years and patients >65 years
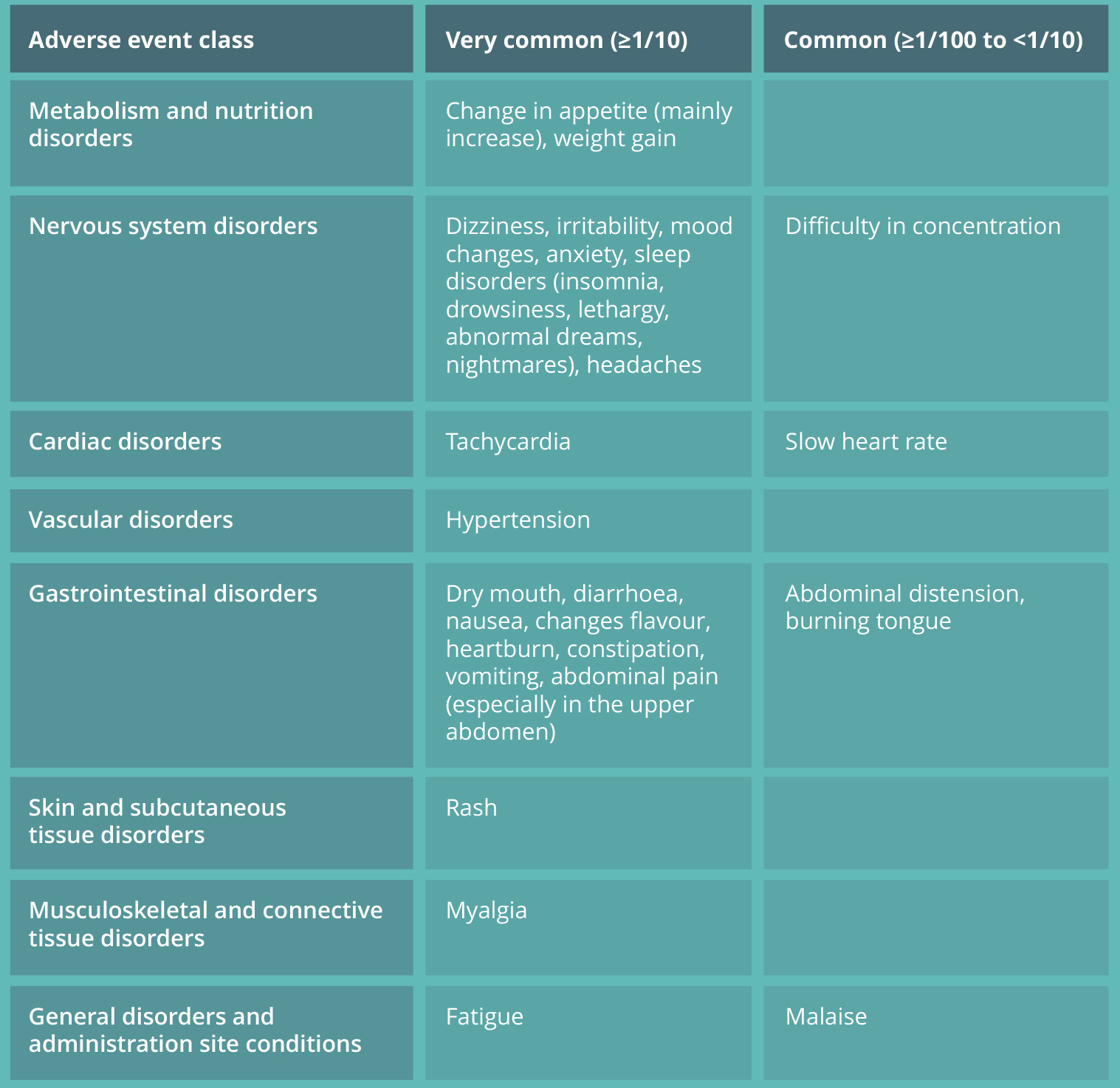
CITIDARON® TOLERABILITY1
- Mild to moderate adverse reactions have been observed with the use of CITIDARON®, most frequently concerning the gastrointestinal tract.
- Clinical trials and prior experience indicate that the majority of adverse reactions occurred at the beginning of CITIDARON® therapy and resolved during treatment.
The following more frequently noted adverse events were observed in clinical trials.
References
- CITIDARON® Summary of Product Characteristics, Consilient Health 2023.

Pharmacovigilance or Medical enquiries please contact:
E: drugsafety@consilienthealth.com
T: (01) 205 7766
All other queries: (01) 205 7760
Healthcare professionals are asked to report any suspected adverse reactions. To report an adverse event or a product complaint about a Consilient Health medicine, please contact Consilient Health at drugsafety@consilienthealth.com or 012057766. Adverse events and product complaints may also be reported to the Health Products Regulatory Authority. Reporting form and information can be found at http://www.hpra.ie then click on “report an issue”.
IE-CH-1564 Date of Preparation April 2024